The H5N1 ‘bird flu’ virus continues to spread among cattle in the US. So far the outbreak has also led to three reported human cases, including one with respiratory symptoms. Meanwhile, H5N1 has been spreading among sea lions and elephant seals in Argentina, with recent genetic and epidemiological analysis suggesting evidence of mammal-to-mammal transmission and occasional mammal-to-bird spillover.
So, how harmful is H5N1 to humans? In news coverage, it is often claimed that around 50% of human H5N1 infections are fatal. This is based on cases and deaths documented to date: as of May 2024, there have been 889 reported H5N1 cases globally, and 463 deaths (i.e. 52% of total).
But this doesn’t mean that half of human H5N1 infections lead to death. Muddling reported symptomatic cases with infections is a common problem when analysing disease data. As Justin Lessler and colleagues previously noted for MERS: ‘Not all persons infected with Middle East respiratory syndrome coronavirus (MERS-CoV) develop severe symptoms, which likely leads to an underestimation of the number of people infected and an overestimation of the severity.’
We saw similar confusion during the COVID-19 pandemic. Around 0.5–1% of infections were estimated to have been fatal in many Western countries (i.e. the infection fatality risk). This infection tally included both those with and without symptoms. Before long, several commentators were comparing this estimate with the ratio of reported seasonal flu cases and deaths, which tends to be around 0.2–0.4% in the US. The claim was that COVID was similar in risk to seasonal flu, and hence nothing to worry about.
But the case fatality risk for flu is not comparable to the infection fatality risk for COVID, because many flu infections will go unreported. A few years ago, we used antibody data to estimate how many people were actually infected with seasonal influenza each year. We estimated 40-50% of younger groups were infected annually, and 15-20% of older groups. Data from a very detailed study in South Africa has since found similarly high values, with at least 35% of participants having a PCR confirmed flu infection during the year. So if we’re calculating the risk that a seasonal flu infection is fatal, we need to compare total flu infections – potentially a third of the population – with annual deaths. Based on the above US values, this would suggest that roughly 0.02-0.05% of flu infections are fatal, i.e. ten times lower than for COVID.
If we want to know the infection fatality risk for H5N1, we therefore need to work out how many infections there have been. And this is where things get tricky. One study of backyard poultry growers in Egypt – which has experienced H5N1 outbreaks – estimated 2% antibody positivity for H5N1 (with 0% positivity among those with no poultry exposure). A subsequent study, which followed growers over time, estimated that we’d expect to see around 17 infections for every 10,000 exposed persons. Yet during 2022, 4,351 people in the US were actively monitored after exposure to H5N1-infected birds. Only one of them subsequently became infected.
In a meta-analysis of available antibodies studies for H5N1, 0.6% of poultry-exposed people in areas with H5N1 outbreaks in poultry had antibodies to H5N1, which can be an indicator of prior infection (the 95% confidence interval was 0.3–0.9%). So there is some evidence that H5N1 infections are happening that aren’t being spotted by routine surveillance. But because estimates are so variable between locations, and reliant on antibody evidence rather than detection of virus by PCR, it’s not easy to extrapolate to converge on a number for how many H5N1 infections there have been globally.
Ideally, we’d be able to derive the infection fatality risk with a calculation like the following:
However, it’s not that simple to estimate how many people globally have been exposed to H5N1. Then there’s the problem that ‘exposed’ can mean different things in different situations, and different types of exposure may come with different levels of risk. Based on the above evidence of undetected infections, the infection fatality risk for H5N1 is likely to be less than 50%, and possibly quite a bit less, but it’s not clear exactly how much.
What’s more, H5N1 deaths may also have gone underreported (e.g. if an H5N1 infection was instead attributed to another seasonal virus). When it comes to calculating the infection fatality risk of H5N1, we’re dividing one number we’re not that confident about by another number we’re really not confident about.
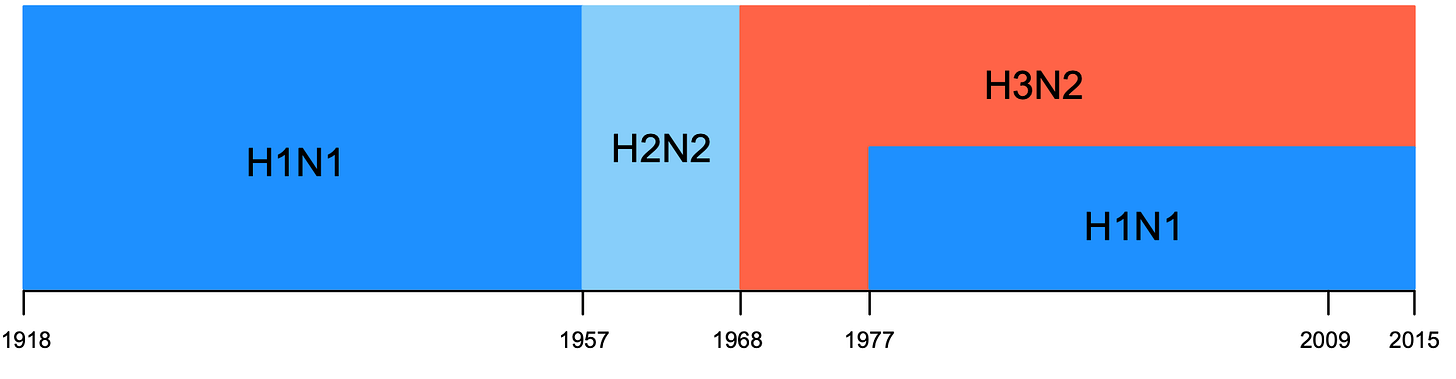
The severity of H5N1 can also depend on what else is circulating within a population. When Katelyn Gostic and colleagues analysed the age distribution of severe H5N1 and H7N9 avian influenza cases, they noticed that the pattern could be explained by what seasonal flu viruses individuals were likely first exposed to. First, here is their plot of which seasonal flu viruses have dominated over time:
So depending on when someone was born, their first infection could have been H1N1, H2N2 or H3N2. This is important because a key protein on the surface of the H5 virus is in the same genetic group as seasonal H1N1 and H2N2, while H7 is in the same group as seasonal H3N2. Their hypothesis was that a primary infection with H1N1 or H2N2 would therefore leave an ‘imprint’ on subsequent immunity, making these age groups less susceptible to avian viruses in the same group (i.e. H5N1). Similarly, primary infection with H3N2 could leave people born after 1968 less susceptible to H7N9.
It turned out these predictions were a good match for the actual pattern of H7N9 and H5N1 cases:
So if we’re discussing how fatal H5N1 is, we don’t just need to consider how many infections (and deaths) may really be out there, undetected. We also need to think about what else has been circulating, and who might end up less susceptible as a result. And, of course, how countries should respond if H5N1 started spreading among humans. Because even if the infection fatality risk turns out to be ‘only’ 10%, or ‘only’ 1%, that would still leave the world with a massive problem.
Cover image source: Thomas Iversen via Unsplash





Very informative for this layperson. Carefully explained. My take is that as a Coronation baby 1953 I MAY have some residual protection to Avian Flu should I need it. That is if age etc doesn't lower defences and I use good hygeine around any occasional bird or infected mammal contacts. Why can't our leaders in the UK message us frankly and help to present science like this. I think more young people would be inspired and become the scientists of the future.
J. McLaren
Nice explanation on a very tricky topic - thank you. The unknowns are overwhelming for estimating a CFR for H5N1 and other bird flues. Today's "leaked" CDC analysis of the effective dose needed for H5N1 antiviral meds adds to the mystery that we are facing. Stay safe!