How we got here with mpox
And where the outbreaks might be going
Earlier this week, WHO declared the spread of mpox in the Democratic Republic of the Congo (DRC), plus a growing number of other countries in Africa, a public health emergency of international concern (PHEIC). This followed an earlier declaration in 2022, when mpox spread widely around the world. But how did we get to the point? And where might things be going next?
A decade ago, I was working quite a lot on mpox analysis. My research at the time focused on infections that spilled over from animals into human populations, from avian influenza H5N1 and H7N9 to emerging coronaviruses like MERS.
Once pathogens like mpox got into human populations, they typically stuttered along in highly random but often small outbreaks. That meant we had to come up with innovative ways to extract insights from the limited data available. As well as its historical relation to smallpox, mpox was epidemiologically interesting because it didn’t transmit very efficiently among humans.
Or so we thought.
A new era for an old virus
When mpox started spreading widely in 2022, it brought echoes of another infection where reality had overturned commonly held assumptions. As I told the BBC at the time, we had always thought Ebola was easy to contain, until that wasn’t the case. Pre-2014, Ebola had caused many small outbreaks that were brought under control, before spreading widely across multiple countries in West Africa during 2014–16.
When mpox was first reported in the 1970s, it spread against a background of cross-protective immunity from smallpox vaccination, so its effective reproduction number R (i.e. the average number of people a typical case spread the virus to) was initially very small, and far below the crucial value of 1 required to generate sustained transmission among humans.
But as you might remember from COVID analysis, the effective reproduction number R depends on population susceptibility:
where R0 is transmissibility in a fully susceptible population and S is the proportion of the population that is susceptible to infection.
In the years since the discontinuation of smallpox vaccination following the eradication of that disease, S increased as new unvaccinated children were born, leading to a corresponding increase in R for mpox.
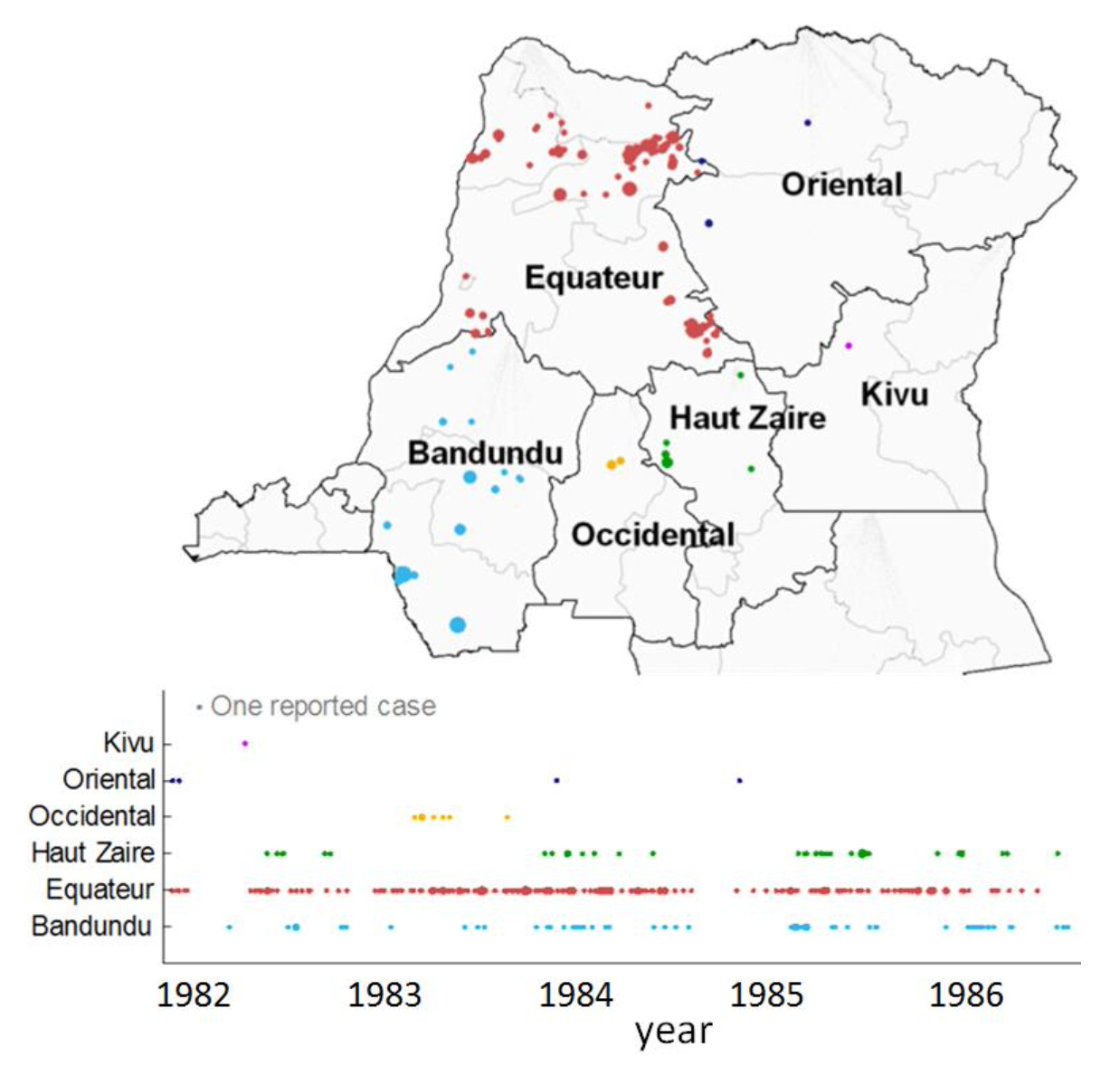
Between the 1980s and 2006-7, there was an estimated 20-fold increase in the rate of new human mpox cases in the DRC. Other outbreaks would follow, with over 100 cases reported in Nigeria in 2017-18. There were also cases identified among travellers or contacts of travellers, like the seven detected in the UK between 2018 and 2021.
While the endemic transmission in DRC and outbreaks in other areas in Central Africa had been caused by the so-called clade I genetic variant of the virus, outbreaks in West Africa had been the result of the clade II variant. The infection that would cause a widespread global outbreak in 2022 – and spark a WHO declaration of a Public Health Emergency of International Concern – was from clade II (or more specifically, the clade IIb subclade). In contrast, the 2024 PHEIC declaration would involve clade I.

Even if R is below 1 in mostly susceptible populations, as it seemed to be for mpox pre-2022, we can still see large ‘stuttering’ outbreaks occasionally, simply by chance. So when an infection causes an unusually large outbreak, it is important to disentangle effects of: chance events; a change in virus characteristics (e.g. via evolution); and a change in the population in which the virus is spreading.
In 2022, the population involved made a big difference, with mpox transmission concentrated within densly connected subset of men who have sex with men (MSM). This is illustrated by the below estimates of how R0 varies with the secondary attack rate (i.e. the per-contact transmission risk), based on MSM and non-MSM sexual contact patterns. It means an mpox infection that might struggle to spread among close sexual contacts in the wider population could have an R0 above the crucial value of 1 in an MSM subpopulation.

At a time in 2022 when only 728 cases had been reported in non-endemic countries, my colleagues concluded that:
without interventions or changes to sexual behaviour, a major outbreak in the MSM population (defined as ≥ 10,000 cases excluding initial cases) is highly likely given the current outbreak size.
Eventually, almost 100,000 cases would be reported globally in that clade II outbreak.
In response to growing case numbers, many countries implemented measures like isolation, contact tracing and vaccination. Historically, it has been thought that because mpox has distinctive symptoms, a long-ish serial interval (i.e. delay between symptoms appearing in case and person they infect), and transmission occurs mostly after symptoms, isolation and contact tracing should be very effective for mpox. In this situation, the main variable influencing control would therefore be the proportion of contacts of cases that can be traced. And if targeted vaccination is planned, the effectiveness of this approach will also depend on the proportion of contacts that can be traced.
Unfortunately, historical assumptions did not always hold up in 2022. Although 94% of patients in a Belgian cohort displayed characteristic skin lesions, a detailed analysis of UK data that linked cases and contacts suggested the majority of cases transmitted before they became symptomatic, considerably more than previously thought.
By late September 2022, around 40,000 vaccine doses had been distributed to at-risk groups in the UK. However, the outbreak peaked in July 2022, before the vaccine rollout had fully got going:

A later analysis of underlying transmission drivers suggested that this outbreak decline was likely to be down to accumulation of post-infection immunity in the groups connected to the outbreak, combined with behaviour change reducing onwards transmission:
Our model-based analysis suggests that the most likely reason the Mpox epidemic in the United Kingdom turned over was a combination of high population exposure among the small number of people in the most sexually active groups and behaviour change resulting in relatively lower risk of forward transmission from infected people.
A new PHEIC
Which brings us to 2024. Although outbreaks have spread in recent months, the threat has been looming for a while. During the 2022 clade II outbreak, there were 200 deaths reported in countries that had not historically reported mpox outbreaks. Yet during the 2023 clade I outbreak in the DRC, there were more than 1,200 deaths reported, and for the first time for clade I, sexual contact was estimated to be a key driver of transmission. In 2024, researchers also identified a new ‘clade Ib’ subvariant that was behind much of the sustained transmission in DRC. Up to the end of July 2024, an additional 2600 confirmed cases and 450 deaths were reported, with 68% of the cases and 85% of the deaths among under 15s.
It’s unlikely this fatality pattern is just down to population age structure; earlier this year, an analysis of systematic reviews of historical clade I outbreaks estimated a much higher fatality risk among the youngest age groups:

There’s also the question of how widespread transmission might be. For example, on 25th July 2024, Burundi reported a new clade Ib mpox outbreak. By 14th August, 23 out of 49 districts were affected, with 103 confirmed cases in total (and 464 suspected).
Then there’s the issue of ongoing clade Ia and clade IIb transmission. For example, between May and July 2024, South Africa reported 22 cases and 3 deaths of clade IIb. Because the fatality risk for this clade is comparatively low, a pre-print published earlier this week estimated that the true outbreak size could be as large as 290–560 cases among MSM in the country.
Where next?
The mpox situation is moving fast, but here are some initial areas where progress would be helpful for the response:
Untangle differences between mpox clades and subclades in terms of what is driven by inherent infection properties and what is shaped by the populations in which they’re spreading. Although there is some evidence from animal experiments, there is still considerable uncertainty about how these infections will behave in different populations, from transmission routes to disease severity. Given the apparent high severity of clade I among children, we should be particularly concerned about what happens if the infection gets into these age groups in other locations, and how easily transmission could persist given children’s relatively high numbers of social contacts.
Understand the true distribution of infection. How many infections are being missed? Which new populations might be at risk (even if historically not thought to be)? Although Sweden reported the first imported case of clade Ib yesterday, it’s likely that infections are already in other countries undetected. Identifying outbreaks will be particularly difficult if hard-to-reach groups are at risk and wider stigma makes people reluctant to report symptoms. In countries where mpox is not yet endemic, there is also the risk of the virus getting into wildlife and establishing reservoirs of infection (despite its original name ‘monkeypox’, mpox is thought to spread mostly among small mammals like rats and squirrels1).
Ensure that available options are used effectively. Vaccine doses are currently concentrated in countries with high wealth and relatively few mpox cases, making them hypothetically rather than actually useful. The experience from 2022 also suggests that vaccination alone will not be enough to curb transmission: prompt non-pharmaceutical measures like isolation and contact tracing, as well as risk communication and behaviour change, will be important to reduce the chances of the infection spreading further.
In many ways, outbreaks like mpox can be thought of as a test for the next major pandemic. Will the global response be effective and equitable? Or will it be late, disjointed and misdirected? After all, if we can’t tackle a relatively slow and symptomatic infection like mpox, with a vaccine already ready and waiting, what hope do we have for a severe outbreak of a novel and highly transmissible flu virus?
Cover image credit: NIAID.



Many thanks for such an informative overview. I’m now so much clearer as to what the issues are.
Adam, I am sorry that you received such an unnecessary threat on twitter.